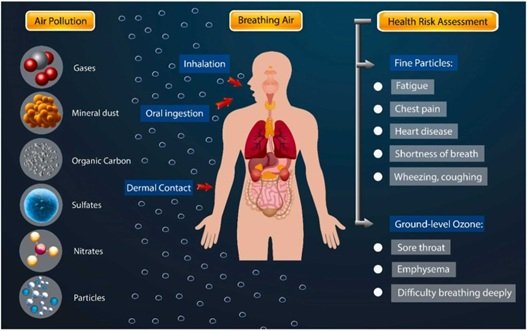
Inhalation studies play a critical role in modern toxicology and drug development by helping scientists predict potential human risks associated with airborne substances. From environmental pollutants to pharmaceutical aerosols, inhalation exposure can pose significant health hazards. Conducting these studies allows researchers to evaluate the safety of substances before human exposure, ensuring regulatory compliance and public health protection.
The primary goal of inhalation studies is to understand how inhaled substances interact with the respiratory system and, subsequently, with the body as a whole. These studies are typically conducted in preclinical models, such as rodents or non-human primates, which provide valuable insights into absorption, distribution, metabolism, and excretion (ADME) of inhaled compounds.
Mimicking Real-World Exposure
One major advantage of inhalation studies is their ability to mimic real-world exposure scenarios. Unlike oral or intravenous studies, inhalation studies consider factors such as particle size, airflow dynamics, and deposition patterns in the respiratory tract. For instance, smaller particles may reach deep into the alveoli, while larger particles may deposit in the upper airways. Understanding these dynamics is crucial because the site of deposition often determines the type and severity of toxic effects.
Evaluating Acute and Chronic Effects
Inhalation studies also allow for the assessment of both acute and chronic exposures. Acute studies help identify immediate toxic responses, such as irritation, inflammation, or respiratory distress. Chronic studies, on the other hand, evaluate long-term effects, including fibrosis, tumor formation, or progressive lung disease. By combining data from both types of studies, scientists can develop a more comprehensive picture of the risks associated with a particular substance.
Controlled Exposure and Regulatory Standards
Another critical aspect of inhalation studies is the ability to use controlled exposure systems. These systems ensure that animals are exposed to precise concentrations of the substance for defined durations, improving the accuracy and reproducibility of results. This level of control also enables the evaluation of dose-response relationships, which are essential for establishing safe exposure limits for humans. Regulatory agencies, such as the Environmental Protection Agency (EPA) and the Food and Drug Administration (FDA), often rely on these studies to set occupational and environmental safety standards.
Supporting Pharmaceutical Development
In addition to identifying potential hazards, inhalation studies are essential for guiding the development of safer pharmaceuticals. For drugs intended for pulmonary delivery, such as inhalers or nebulizers, these studies provide insights into efficacy, absorption, and local tolerability. Researchers can assess whether a compound reaches the intended site of action in the lungs and whether it causes any unintended respiratory damage. Integrating data from these studies with other preclinical models allows for a more informed prediction of human responses. This process often involves using preclinical efficacy testing to balance safety and therapeutic potential before advancing to clinical trials.
Informing Environmental and Occupational Safety
Furthermore, inhalation studies contribute to risk assessment for environmental and occupational exposures. Workers in industries such as chemical manufacturing, agriculture, or mining may be exposed to airborne chemicals regularly. Inhalation studies help identify which substances pose the highest risk and guide the implementation of protective measures, such as ventilation systems, personal protective equipment, and exposure limits. Similarly, these studies inform public health decisions regarding air quality standards and pollution control.
The Future of Inhalation Risk Assessment
While no animal model can perfectly replicate human physiology, inhalation studies remain one of the most reliable tools for predicting human risks. Advances in technology, including sophisticated inhalation chambers, imaging techniques, and computational modeling, continue to enhance the accuracy of these studies. By integrating experimental data with predictive modeling, researchers can better understand how inhaled substances behave in the human body and make more informed safety assessments.
Closing Thoughts
Inhalation studies are indispensable for evaluating the potential risks of airborne substances. They provide critical insights into toxicity, deposition patterns, dose-response relationships, and long-term health effects. From guiding pharmaceutical development to protecting workers and the general public, these studies form a cornerstone of modern toxicology and risk assessment. Through careful design and integration with other preclinical methods, inhalation studies help ensure that new substances are both effective and safe for human use.
Read More –
Golden Triangle Tour Packages for Photography Lovers: A Picture-Perfect Adventure
Cost-Effective Mail Solutions for Small Businesses with PO Boxes